Awards
ISO 13485:2016
*Issue date- Initial Issue Date
- Issuer: DNV Product Assurance AS
- Issue Date: 2003-Nov-17
QMS
- Good Manufacturing Practices
- Issuer: Ministry of Health and Welfare
Japan MHLW
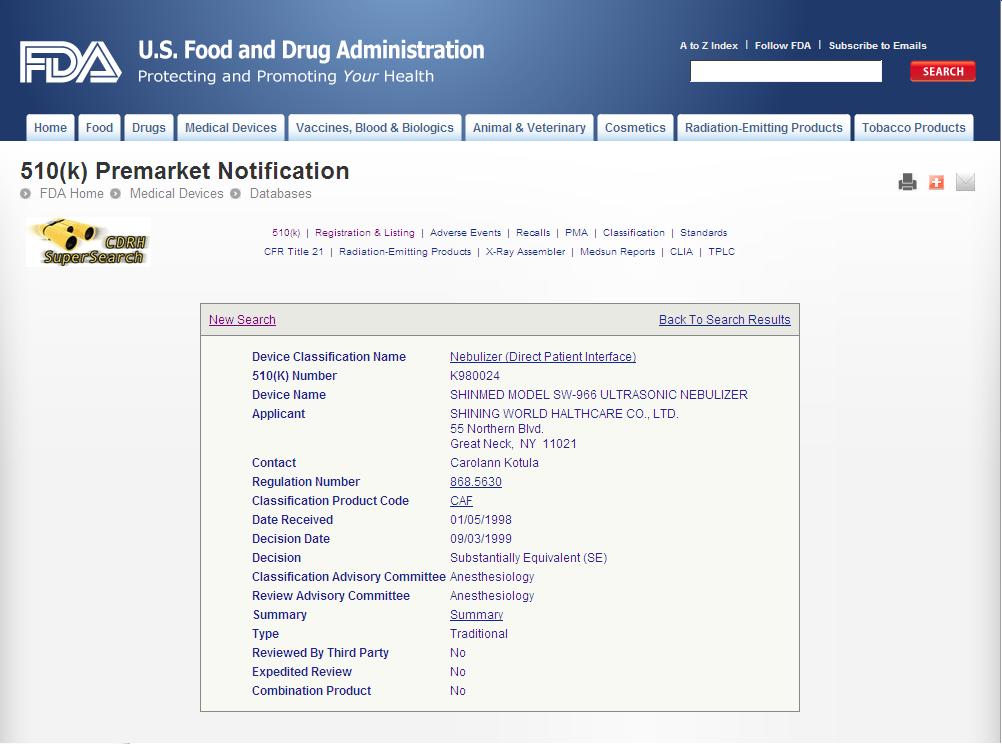
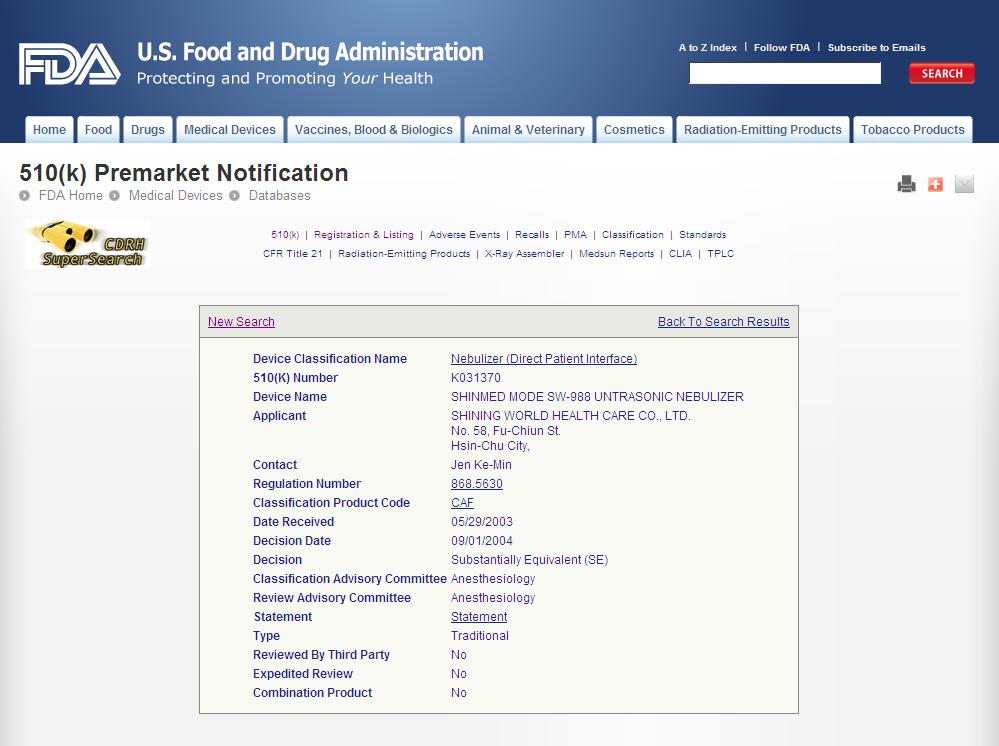
ESU Accessories
Endo hook series
Monopolar cable
- Issuer: Ministry of Health, Labor, and Walfare
- Issue Date: 2010-Mar-11
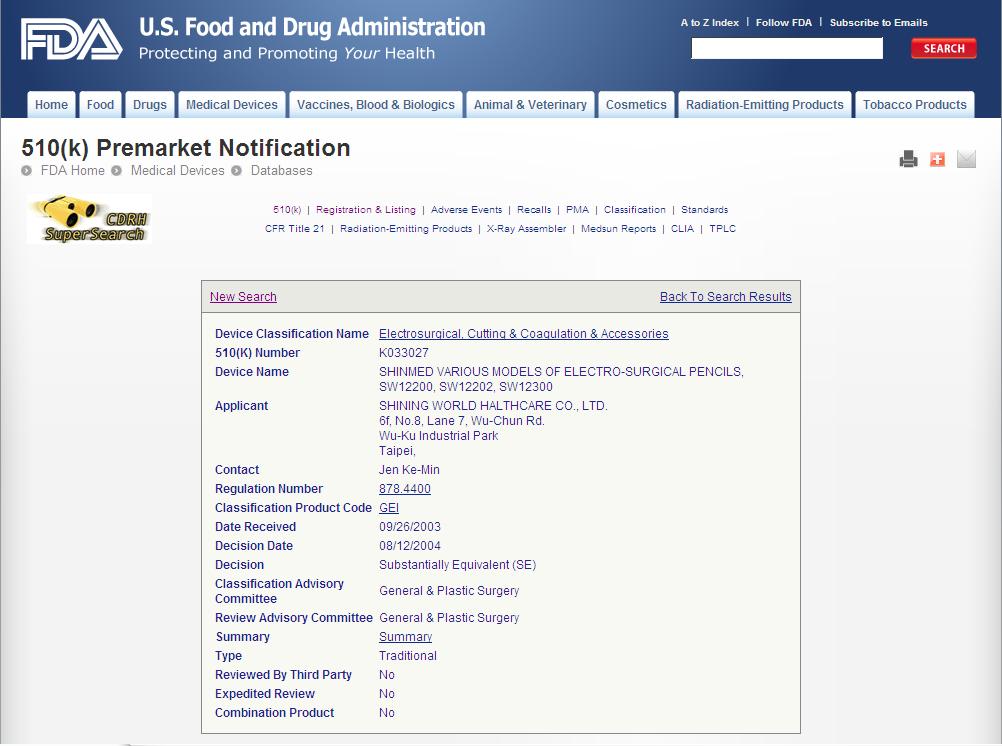
FDA 510K-clearance
Electrosurgical cutting and coagulation device and accessories
- Issuer: FDA, USA
- Issue Date: 2004-Aug-12

FDA 510K-clearance
ShinEvac Smoke Evacuation Pencil- AIO/Classic
- Issuer: FDA, USA
- Issue Date: 2022-Jan-28
Taiwan Excellence Awards
Smoke Evacuation Pencil- ShinEvac Classic
- Issuer: Ministry of Economic Affairs (MOEA), Taiwan External Trade Development Council (TAITRA)
- Issue Date: 2019

D&B TAIWAN’S TOP 1000 ELITE SME AWARDS 2022
D&B TAIWAN’S TOP 1000 ELITE SME AWARDS 2022 HONORS OUTSTANDING SMALL-MEDIUM COMPANIES
SHINMED has been awarded the D&B TOP 1000 ELITE SME AWARDS for the second time!
94.4% of the winners outperformed peers from their own industries, beating larger companies and all SMEs in Taiwan. This award not only acknowledges SHINMED's continuous commitment to providing better products and services to our customers but also highlights our dedication to enhancing our ESG management.
- Issuer: D&B Taiwan
- Issue Date: 2022
Contact Detail
| Contact: | International Sales Team |
| Address: | No. 22, Lane 116, Wugong 2nd. Rd., Wugu Dist., New Taipei City, 24888, Taiwan, R.O.C. |
| TEL: | +886-2-22900966 |
| FAX: | +886-2-22903966 |
| Email: | [email protected] |
| URL: | http://www.shinmed.com |